
Worksheets and No Prep Teaching Resources
Reading Comprehension Worksheets
Healthy Life

Healthy Life
 Worksheets and No Prep Teaching Resources Reading Comprehension Worksheets Healthy Life |
 Healthy Life |
| edHelper's suggested reading level: | grades 6 to 12 | |
| Flesch-Kincaid grade level: | 7.11 |
| Print The Ozone Layer and You (font options, pick words for additional puzzles, and more) |
| Quickly print reading comprehension |
| Print a proofreading activity |
|
The Ozone Layer and You
By Cindy Grigg |

|
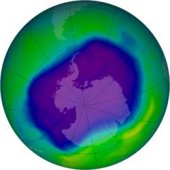 1 There are many stories in the news about the hole in the ozone layer. What is the ozone layer, and why are people so concerned about this problem? Scientists have discovered that the ozone layer is getting thinner. Some predict that the sun's rays will cause a sharp increase in deaths from skin cancer over the next fifty years. These harmful rays can also damage the eyes, create wrinkles, and cause other health problems.
1 There are many stories in the news about the hole in the ozone layer. What is the ozone layer, and why are people so concerned about this problem? Scientists have discovered that the ozone layer is getting thinner. Some predict that the sun's rays will cause a sharp increase in deaths from skin cancer over the next fifty years. These harmful rays can also damage the eyes, create wrinkles, and cause other health problems. |
Create Weekly Reading Books
Prepare for an entire week at once! |
| Leave your feedback on The Ozone Layer and You (use this link if you found an error in the story) |
 |
Healthy Life
|
 |
High School Reading Comprehensions and High School Reading Lessons
|