
Worksheets and No Prep Teaching Resources
Reading Comprehension Worksheets
Earth Science

Earth Science
 Worksheets and No Prep Teaching Resources Reading Comprehension Worksheets Earth Science |
 Earth Science |
| edHelper's suggested reading level: | grades 9 to 10 | |
| Flesch-Kincaid grade level: | 9.53 |
| Print Earth Lab: Atomic Structure (font options, pick words for additional puzzles, and more) |
| Quickly print reading comprehension |
| Print a proofreading activity |
|
Earth Lab: Atomic Structure
By Trista L. Pollard |

|
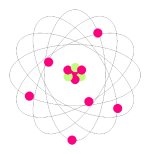 1 When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and green gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imagine gumballs on a circular wire). Most of the atom's mass is found in the nucleus. However, the nucleus's size is extremely small compared to the size of the atom. Most of the atom's volume is empty space. Fast-moving electrons form a cloud around the nucleus. Within this electron cloud, the electrons are spaced at different distances from the nucleus. These areas of electrons are called energy levels or shells. Each shell can only have a certain number of electrons. Since electrons are negatively charged and opposite charges attract, the electrons are attracted to the nucleus, which has a positive charge. It is this attraction that keeps the electrons inside the atom.
1 When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and green gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imagine gumballs on a circular wire). Most of the atom's mass is found in the nucleus. However, the nucleus's size is extremely small compared to the size of the atom. Most of the atom's volume is empty space. Fast-moving electrons form a cloud around the nucleus. Within this electron cloud, the electrons are spaced at different distances from the nucleus. These areas of electrons are called energy levels or shells. Each shell can only have a certain number of electrons. Since electrons are negatively charged and opposite charges attract, the electrons are attracted to the nucleus, which has a positive charge. It is this attraction that keeps the electrons inside the atom.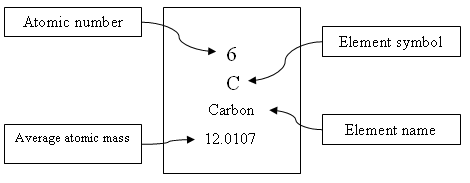
 |
Create Weekly Reading Books
Prepare for an entire week at once! |
| Leave your feedback on Earth Lab: Atomic Structure (use this link if you found an error in the story) |
 |
Earth Science
|
 |
High School Reading Comprehensions and High School Reading Lessons
|