
Worksheets and No Prep Teaching Resources
Reading Comprehension Worksheets
Earth Science

Earth Science
 Worksheets and No Prep Teaching Resources Reading Comprehension Worksheets Earth Science |
 Earth Science |
| edHelper's suggested reading level: | grades 9 to 11 | |
| Flesch-Kincaid grade level: | 11.12 |
|
The Geometry of Minerals
By Trista L. Pollard |

|
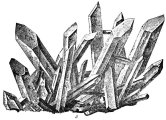 1 Deep inside the Earth's minerals lies a little bit of geometry. Every mineral has a specific crystalline structure. This geometric structure is the result of the careful arrangement of the minerals' atoms, ions, or molecules. It is this specialized geometric structure that helps scientists to categorize the Earth's minerals.
1 Deep inside the Earth's minerals lies a little bit of geometry. Every mineral has a specific crystalline structure. This geometric structure is the result of the careful arrangement of the minerals' atoms, ions, or molecules. It is this specialized geometric structure that helps scientists to categorize the Earth's minerals. |
Create Weekly Reading Books
Prepare for an entire week at once! |
| Leave your feedback on The Geometry of Minerals (use this link if you found an error in the story) |
 |
Earth Science
|
 |
High School Reading Comprehensions and High School Reading Lessons
|